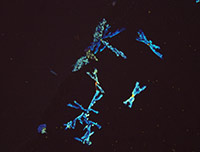
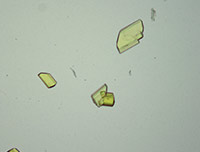
Library of Microcrystalline Tests for Novel Psychoactive Substances
Authors: Matthew Quinn, B.S.; Michael Cain Jr., B.S. and Monica Joshi, Ph.D.
Corresponding author:
Monica Joshi, Ph.D.
Department of Chemistry
West Chester University of Pennsylvania
mjoshi@wcupa.edu
Citation: Quinn, M., Cain Jr., M. and Joshi, M. Library of Microcrystalline Tests for Novel Psychoactive Substances
Date first published: June 30, 2017
This project was supported by Award No. 2014-R2-CX-K008, awarded by the National Institute
of Justice, Office of Justice Programs, U.S. Department of Justice. The opinions,
findings, and conclusions or recommendations expressed in this publication are those
of the authors and do not necessarily reflect those of the Department of Justice.
Introduction
A microcrystalline test is a precipitation reaction between a drug and a reagent, forming an insoluble drug-reagent complex that is unique to that specific test. These tests are quick, non-destructive, and can be used as preliminary and confirmatory tests with expertise. Microcrystalline tests are one of the oldest analytical chemistry practices and their use for classic drugs such as cocaine, heroin and amphetamines is well documented. However, there is very limited research on microcrystalline tests for the novel compounds encountered by law enforcement today. This library is an effort to increase understanding and promote use of microcrystalline tests for novel psychoactive substances.
The 30 substances included in this study represent the ever evolving structural classes of cathinones, phenethylamines, piperazines, aminoindanes and opioids. The page for each substance includes descriptions and photomicrographs for characteristic crystals. Crystal formation time and sensitivity vary widely between classes of substances and within a class. Characteristic crystals were observed with substance test amounts ranging between 1-30 µg of test substance. The microcrystalline tests that performed reliably with a practical crystal formation time are reported in the library. Infrared spectroscopy is a well-established technique for the identification of drugs and adulterants and is categorized as a SWGDRUG category A technique for structural identification. Microcrystalline tests are categorized as category B technique. Infrared microspectroscopy of microcrystals adds structural information to the crystals observed. The library presents infrared spectra collected for microcrystals of novel psychoactive substances. The infrared spectra demonstrate that the microcrystal observed with a specific reagent is indeed representative of the structure of the substance being studied.
The purpose of the library is to serve as a reference to analysts performing microcrystalline tests. We invite analysts working with typical forensic samples to provide feedback on the performance of the tests and any deviations from crystals observed with reference chemicals.
Procedures and Instrumentation
Chemical reference standards for all substances were purchased from Cayman Chemical (Ann Arbor, MI) a 1 mg/mL methanolic solutions or as 1 mg solids. The solids were dissolved in 1 mL methanol for the study. Reagents used in this study were made as per the recipes listed below. A drop of test solution was placed on a glass slide to achieve a dried residue amount between 1- 30 µg depending on the analyte. The residue was dissolved in 5-10 µL of water, 10% hydrochloric acid or 10% acetic acid solutions. To this drop, 10 µL of reagent was added and the drop mixed to stimulate crystal growth. Microcrystal formation time, shape, habit and features under crossed polars were observed and documented.
A Leica DM 750P polarized light microscope and an Olympus BX43F polarized light microscope were used to observe the resulting microcrystals. Characteristic microcrystals were documented as photomicrographs in the Olympus cellSense Entry imaging software at 100-200x magnification. A Thermo Scientific™ Nicolet™iN™10 Infrared Microscope with a liquid nitrogen cooled mercury, Cadmium, Tellurium (MCT) detector was used for analysis. All data was processed and analyzed in the Thermo Scientific™ OMNIC™ Picta™ software. The spectra were collected in transmission mode on AMTIR windows. Crystals grown on glass slides were carefully moved to the AMTIR windows with Microtools(MiTeGen LLC, Ithaca, NY). Excess reagent was wicked away with fine paper wicks and the crystals were washed with chloroform. This procedure removed interferences in the spectra and improved the quality of the infrared spectrum collected for a microcrystal.
Reagents evaluated
- Gold chloride: Two formulations were used
- 5% Aqueous HAuCl4
- 5% HAuCl4 in 1:2 concentrated H2SO4: H20
- Gold bromide (HAuBr4): 1 g HAuCl4 + 0.76g NaBr in 5 mL glacial CH3COOH + 15 mL 2:3 concentrated H2SO4: H20
- Platinic chloride (H2PtCl6): 5% Aqueous H2PtCl6
- Platinic bromide (H2PtBr6): 1 g H2PtCl6 in 1.7 mL 40% HBr + 20 mL 2:3 concentrated H2SO4: H20
- Mercuric chloride (HgCl2): Two formulations were used depending on the length of time for crystal growth. The reagent crystallizes very quickly in the aqueous formulation and prevents growth of drug-reagent crystals.
- 1 g HgCl2 in 100 mL water
- 1 g HgCl2 2.5 mL 1:1 Glycerol: Water 14.2 mL water, 500 μL 3M HCl
- Mercuric iodide (HgI2): 1 g HgI2 in 20ml 27:73 HCl: H20
Novel Psychoactive Substances Studied
- 3,4-Methylenedioxypyrovalerone (3,4-MDPV)
- 1-(4-Methoxyphenyl) piperazine (4-MeOPP)
- 25B-NBOMe
- 2C-B
- 2C-B-BZP
- 2C-B-FLY
- 2-Ethylmethcathinone (2-EMC)
- 3-Ethylmethcathinone (3-EMC)
- 3-Methylbuphedrone
- 4-FluoroBZP
- 4-Fluoromethcathinone
- 4-Methylbuphedrone
- 4'-methyl-α-Pyrrolidinohexanophenone (MPHP)
- 4'-methyl-α-Pyrrolidinopropiophenone (4-MePPP)
- 5-IAI
- Bromo-DragonFLY
- Butylone
- BZP
- Ethcathinone
- Fentanyl
- FIBF
- Furanyl Fentanyl
- MDAI
- Mephedrone
- Methcathinone
- Methylone
- NRG-3
- Pentedrone
- W-18
- α-Pyrrolidinopentiophenone
Additional Resources
- Leonie Elie, Mark Baron, Ruth Croxton, Mathieu Elie, Microcrystalline identification of selected designer drugs, Forensic Science International, Volume 214, Issue 1, 2012, Pages 182-188
- C.C. Fulton, Modern Microcrystal Tests for Drugs, first ed., John Wiley & Sons, Inc., New York, 1969