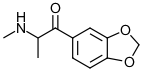
Methylone
5% Aqueous HAuCl4
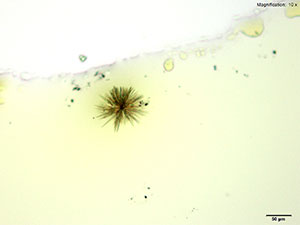
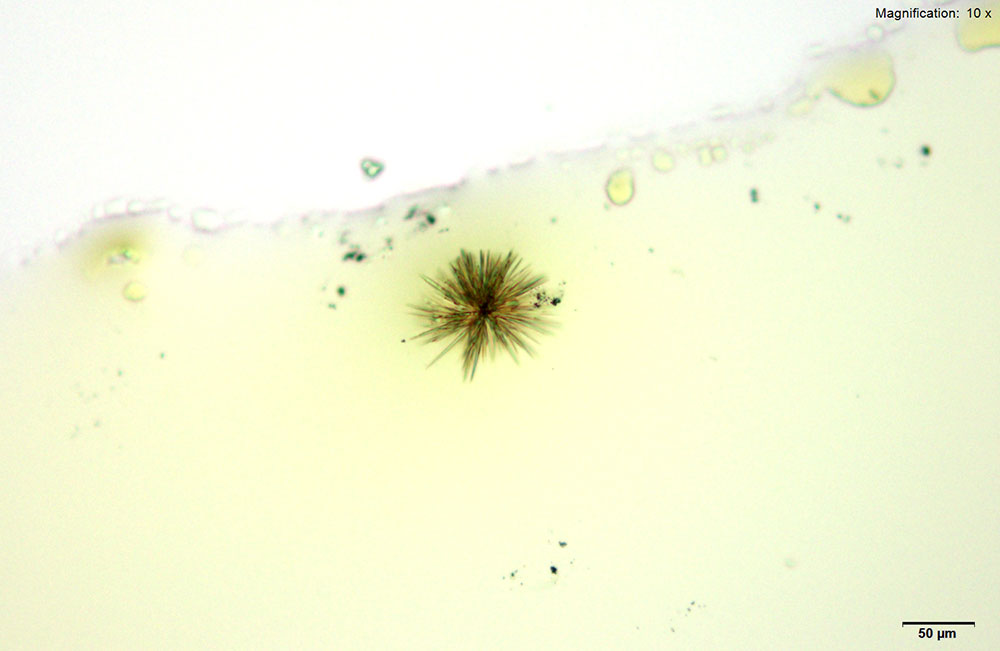
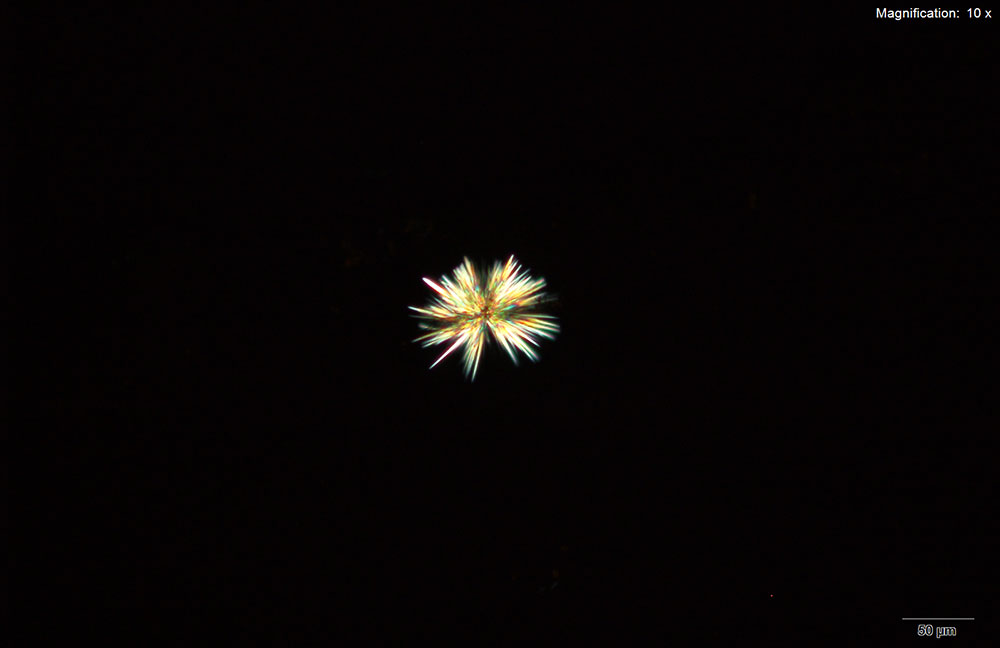
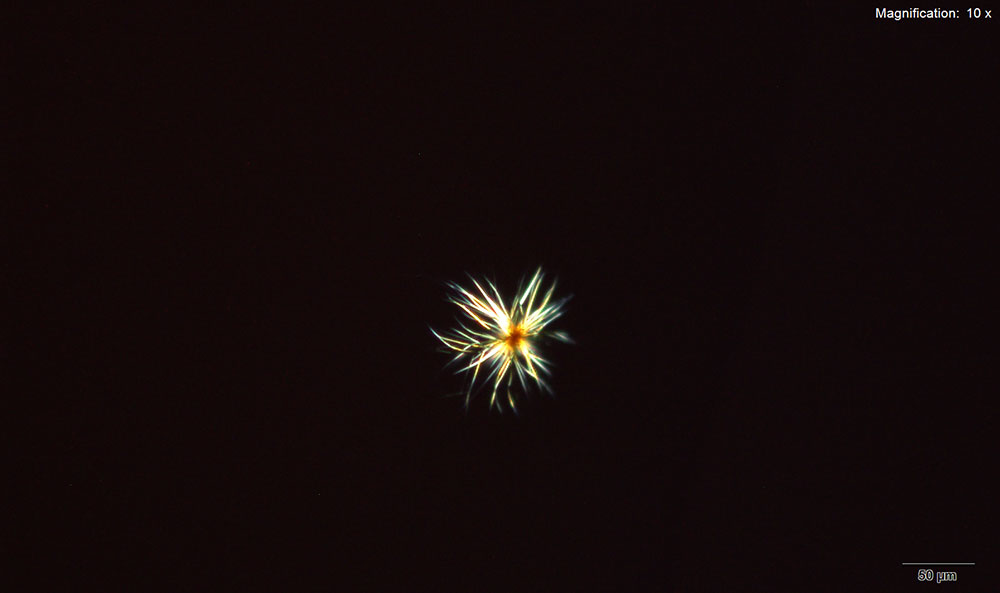
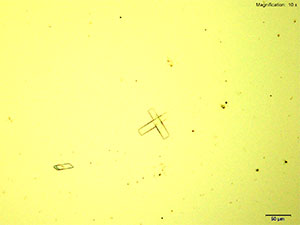
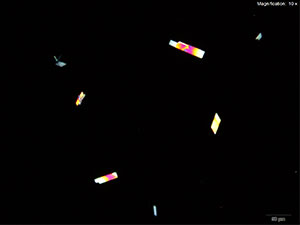
Methylone in 10% hydrochloric acid with gold chloride reagent Methylone in 10% hydrochloric acid with gold chloride reagent gives different sized burrs of colorless needles. The burrs appear dark in the center with colorless sharp needles on the periphery. Under crossed polars, the burrs are very bright and appear to have 4 quardrants in the circle.
Gold Bromide (HAuBr4)
Methylone in gold bromide reagent gives red, wispy hair like crystals that grow as loose sheaves or rosettes. The crystals may be large or small but are easily recognized under crossed polars.
Platinic Chloride (H2PtCl6)
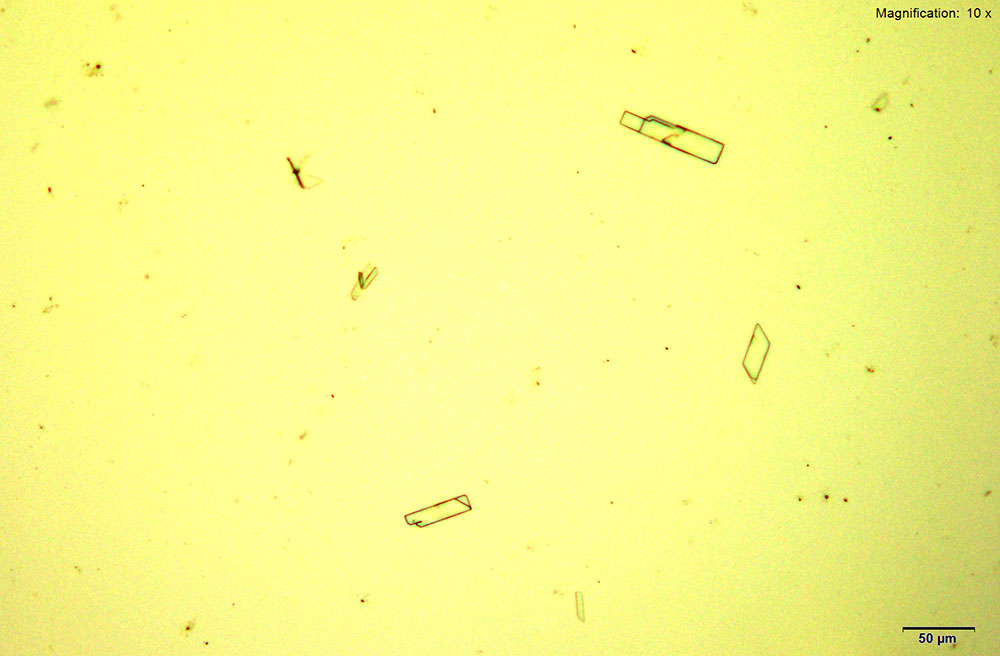
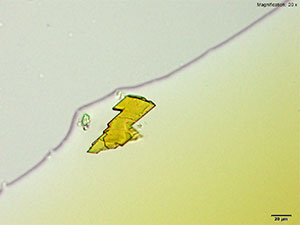
Platinum based reagents are best for methylone and the molecular characterization using infrared microspectroscopy. Methylone in 10% hydrochloric acid with platinic chloride reagent forms colorless rectangular plates and tablets that may grow individually or in tufts. The crystals grow abundantly and as composites with methylone amounts greater than 10 μg.
IR Spectrum - Platinic Chloride (H2PtCl6)
Platinic Bromide (H2PtBr6)
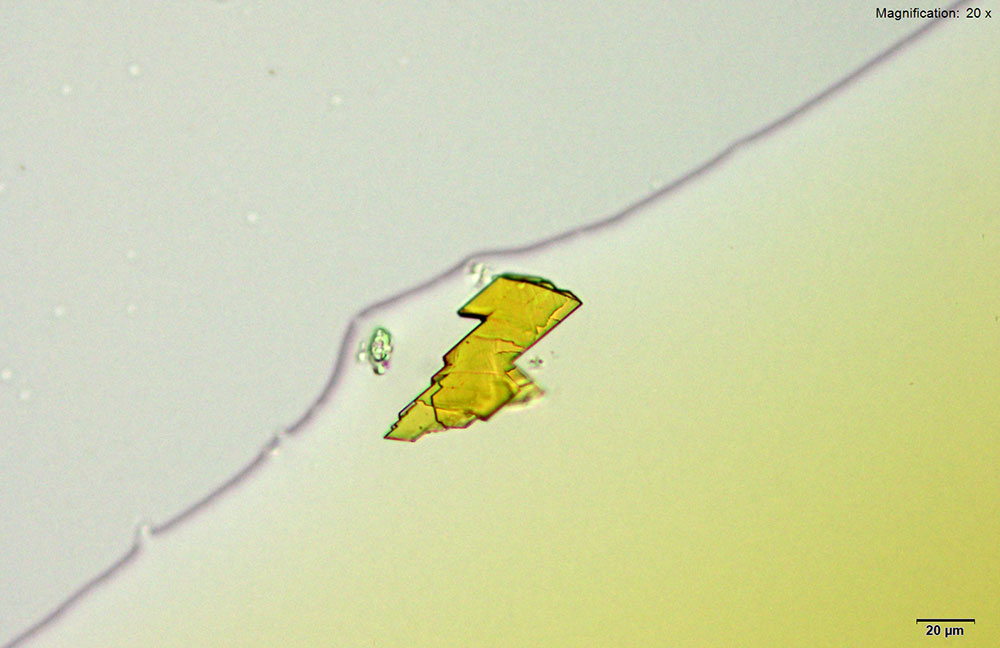
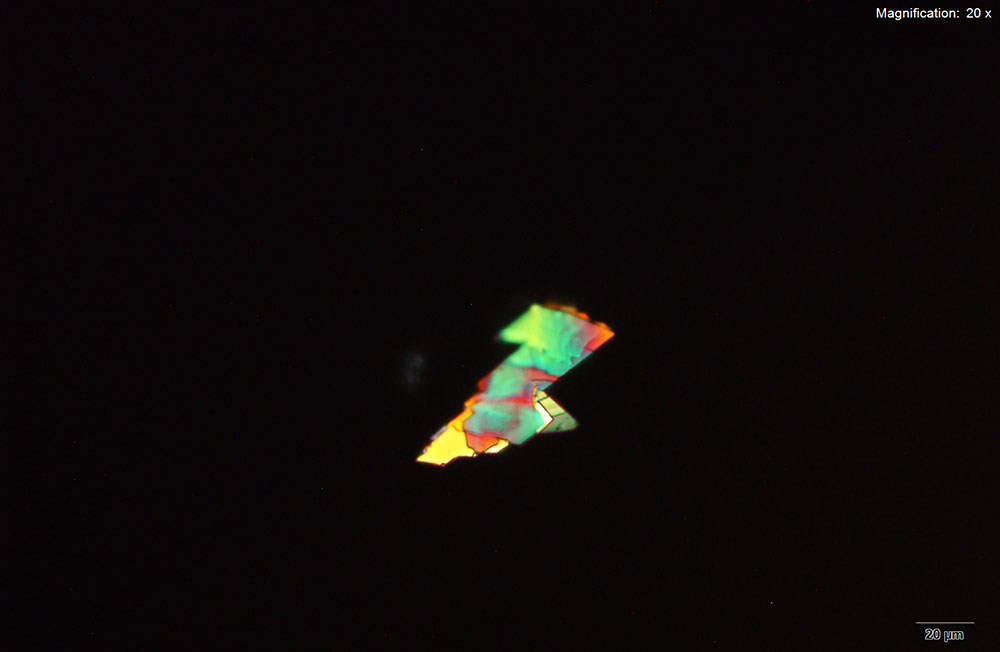
Crystals for methylone in platinic chloride reagent are thick, yellow prisms and tablets that grow as clusters. The crystals grow large with characteristic features with methylone amounts lower than 10 μg.
The large crystals are suitable for infrared spectroscopy and give information rich spectra when isolated from the reagent.