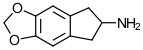
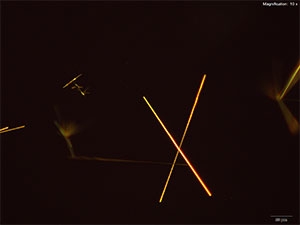MDAI
Platinic Chloride (H2PtCl6)
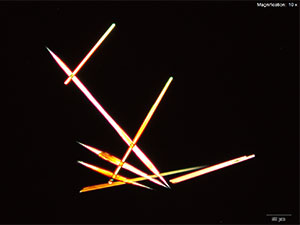
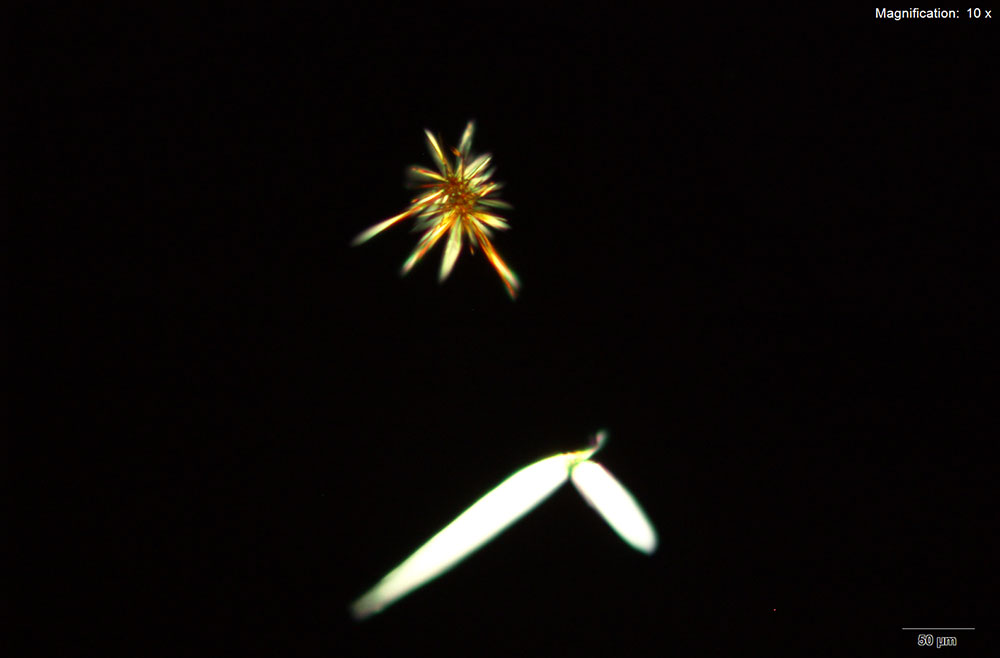
MDAI forms thin flat blades with platinic chloride reagent. With water as the solvent, the crystals are arranged as clusters of thin yellow blades with pointed tips. These blades appear bright yellow under crossed polars. The blades grow in loose clusters with longer blades in hydrochloric acid. With platinic chloride reagent and MDAI dissolved in 10% acetic acid, the blades in the cluster are broader and larger.
The clusters hold together very well and can be transferred for infrared microspectroscopy.
IR Spectrum - Platinic Chloride (H2PtCl6)
Platinic Bromide (H2PtBr6)
With platinic bromide reagent, long, smooth needles arranged in clusters or individually grow abundantly. These crystals are bright under crossed polars are very characteristic for MDAI.