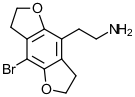
2C-B-FLY
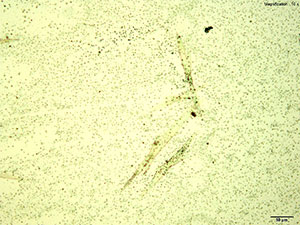
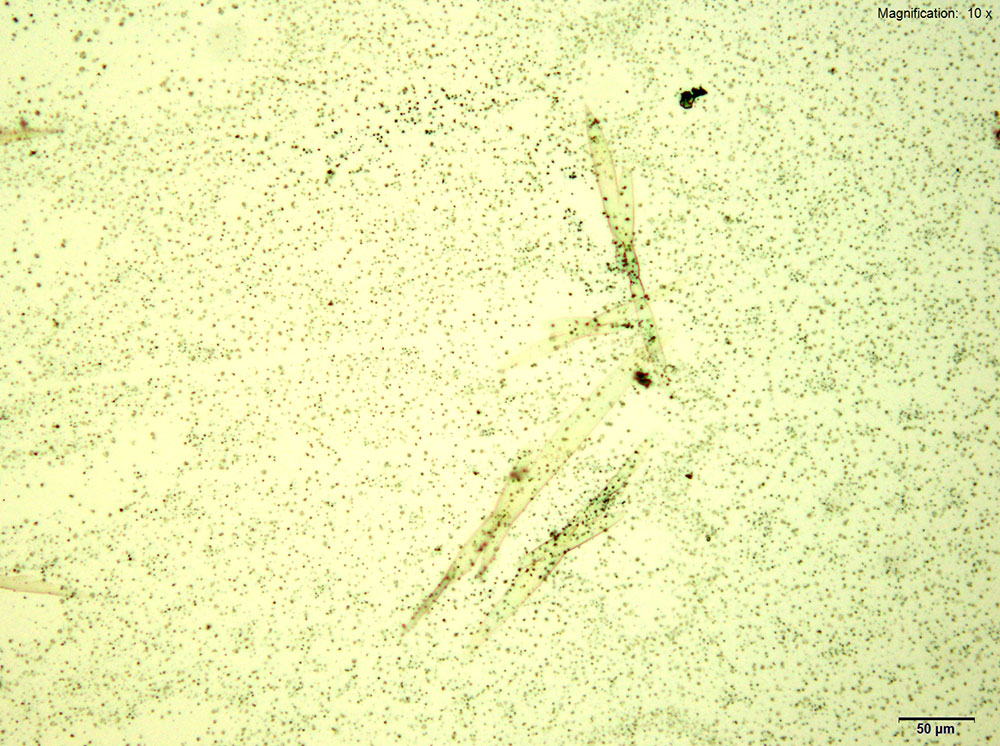
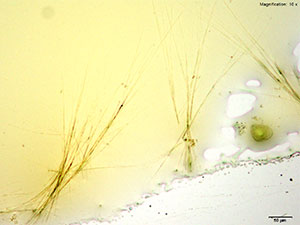
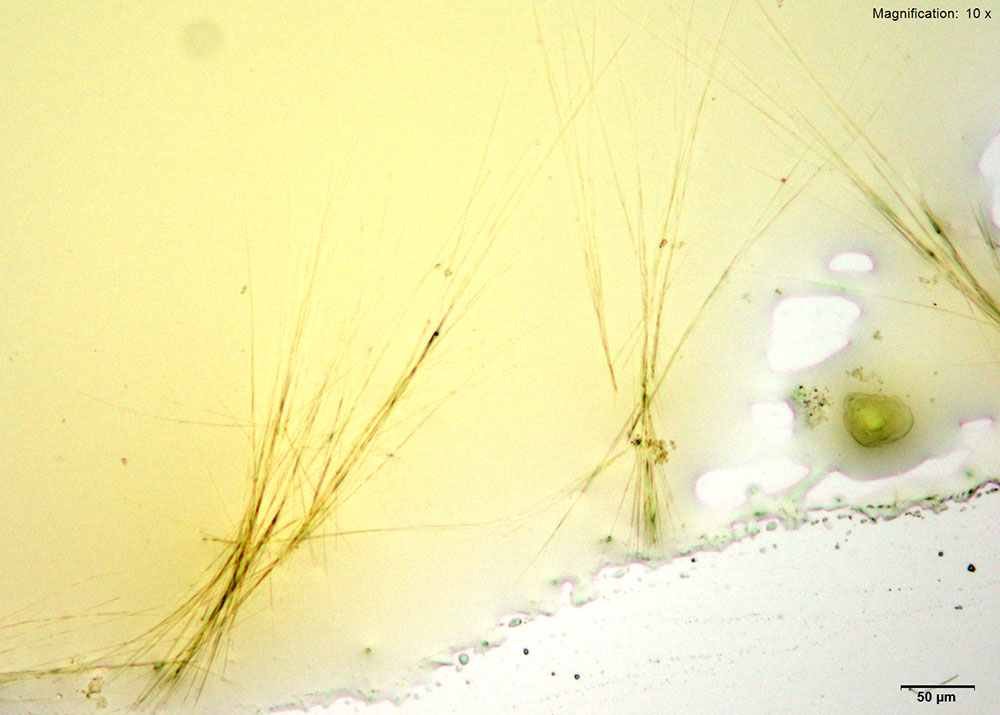
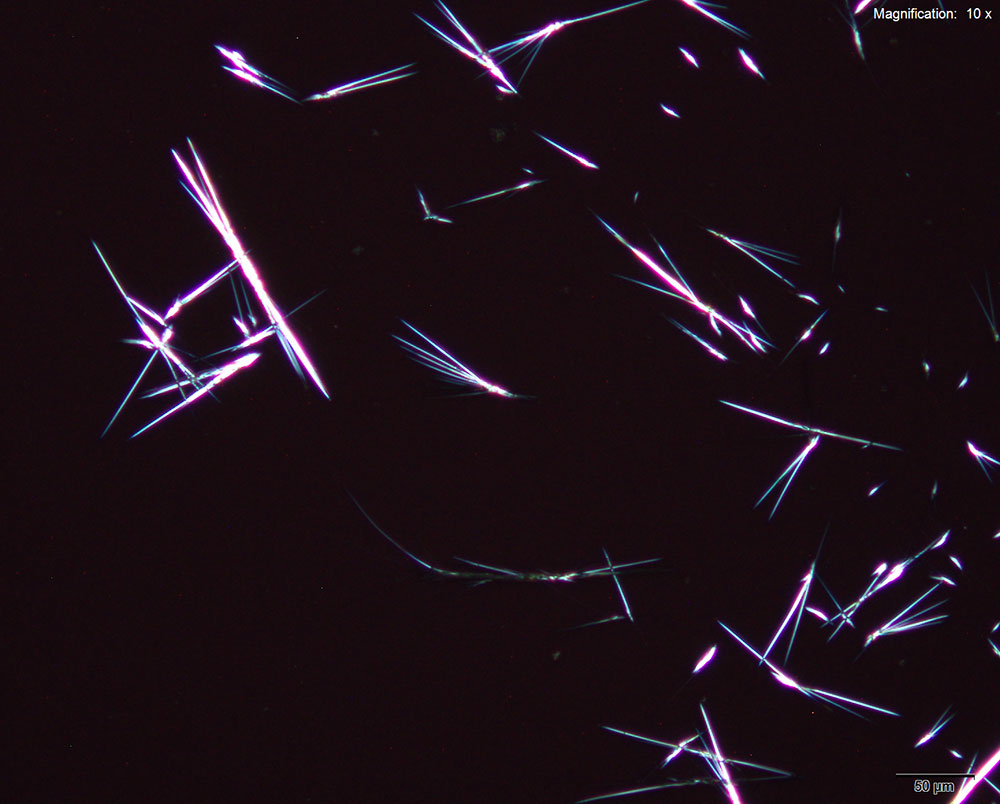
5% Aqueous HAuCl4
2C-B-FLY in 10% hydrochloric acid makes characteristic long red blades with a tapered growing end. The blades are very bright and yellow-orange in color under crossed polars. Crystals are observed when 2C-B-FLY is dissolved in water or in 10% acetic acid crystal formation is slow and inconsistent showing none of the features characteristic of hydrochloric acid.
IR Spectrum - 5% Aqueous HAuCl4
5% HAuCl4 in 1:2 concentrated H2SO4: H20
In the acidic gold chloride reagent, 2C-B FLY makes colorless, flat and long blades that grow individually or in X-shaped habit. The colorless blades are not very apparent in brightfield visualization but are very bright under crossed polars.
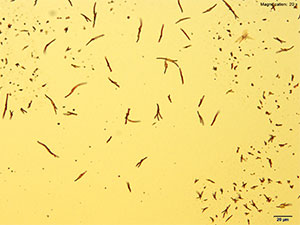
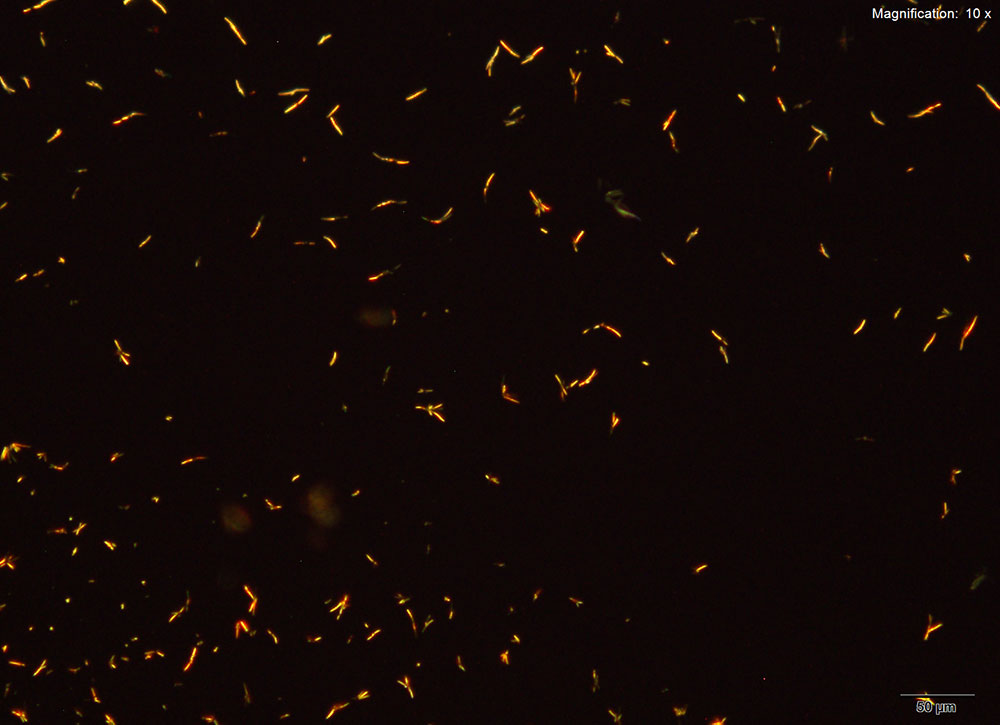
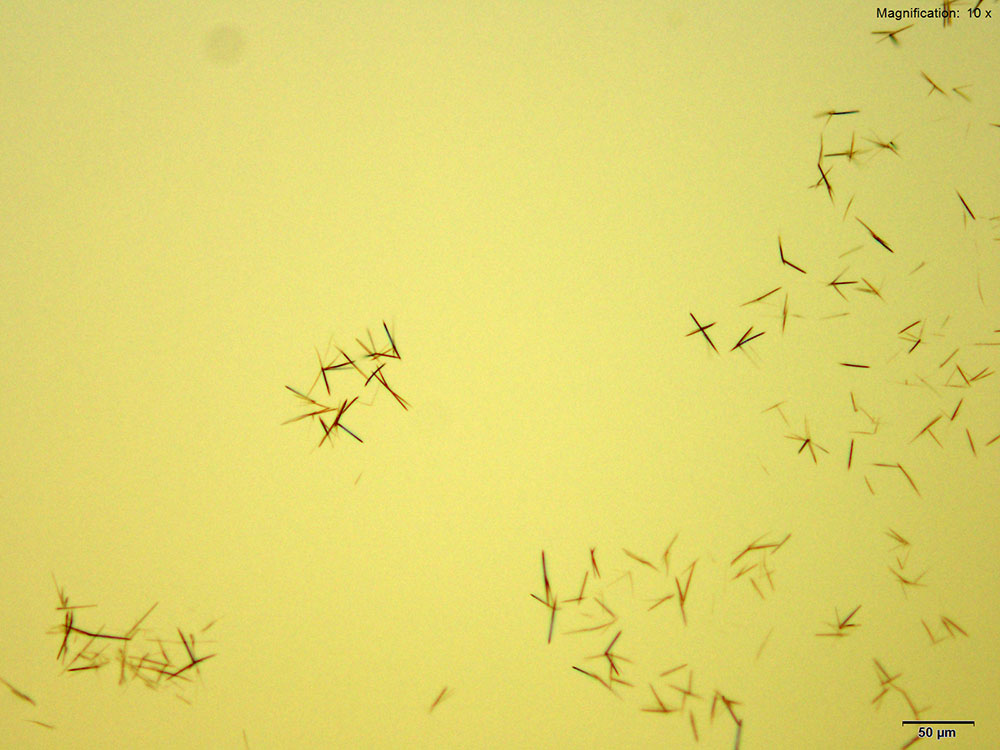
Gold Bromide (HAuBr4)
With gold bromide reagent, very small red rods are observed for 2C-B-FLY. These rods grow abundantly and the drop may appear to have precipitated immediately upon adding reagent. The wavy short rods are better visualized under higher magnifications.
Platinic Chloride (H2PtCl6)
Long colorless-yellow needles are observed for 2C-B-FLY with water and 10% acetic acid. When dense clusters of these long wispy needles are formed, the clusters appear dark. The needles are very bright under crossed polars. When 10% hydrochloric acid is used to dissolve the drug for the test, the crystals formed are darker, shorter and broader.
Platinic Bromide (H2PtBr6)
Clusters of short red needles are observed for 2C-B-FLY with platinic bromide. The light clusters grow abundantly. Under crossed polars, the crystals are bright but their visibility and color is dependent on their orientation in the cluster.
Mercuric Chloride (MgCl2)
Mercuric chloride reagent gives long, flat and thin blades that may grow individually or in clusters. The colorless blades when oriented on their sides look like dark needles and appear dark. The blades appear white and bright under crossed polars.
Mercuric Chloride (Mgl2)
Crystals observed for mercuric iodide are feathery clusters of short needles. The needles appear bright under crossed polars.