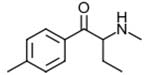
4-Methylbuphedrone
5% HAuCl4 in 1:2 concentrated H2SO4: H2O
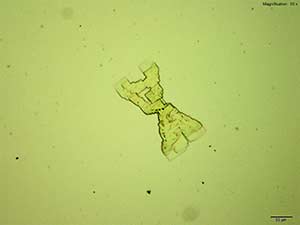
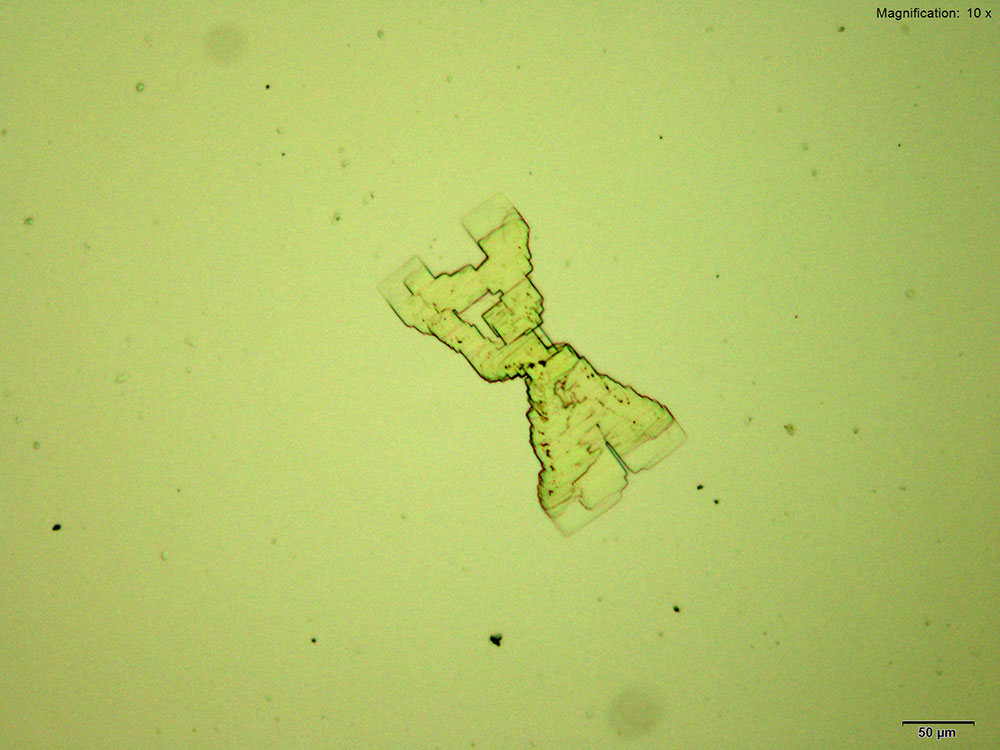
4-Methylbuphedrone forms colorless thick tablets that are arranged as X-shaped composites with the acidic gold chloride reagent. The overall formation is quite distinct. The composites are bright and multicolored under crossed polars.
Gold Bromide (HAuBr4)
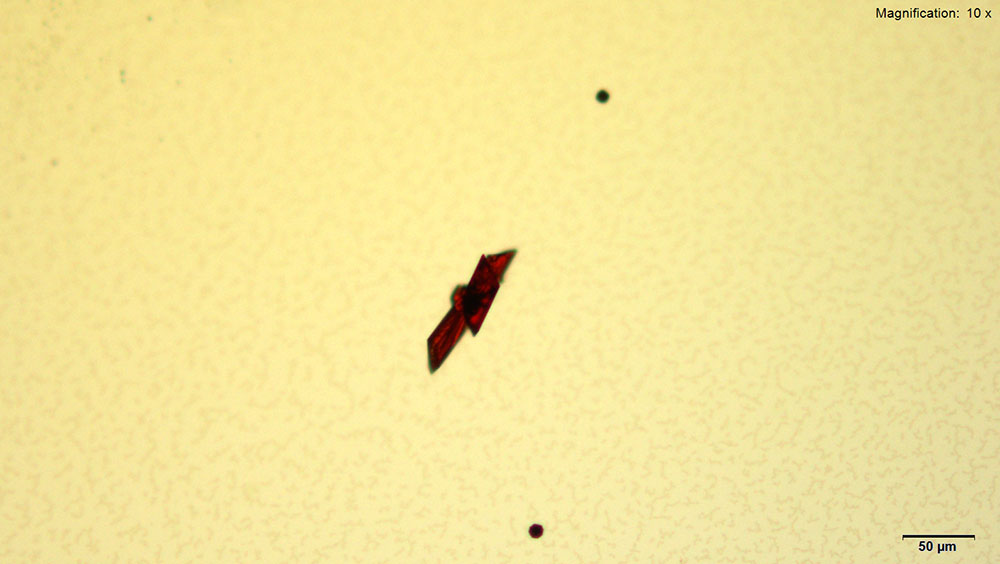
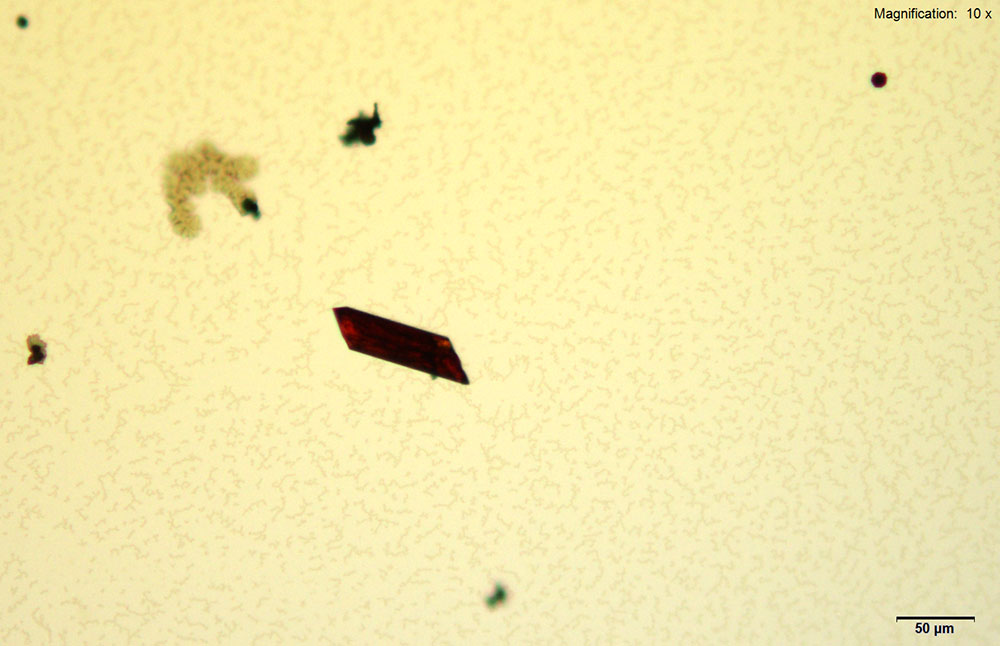
Gold bromide reagent gives distinct red rectangular tablets with 4-methylbuphedrone. The thick tablets grow individually or in pairs. The crystals take long to grow (~30 min) at 4-methylbyphedrone concentrations below 25 μg. The crystals appear bright red under cross polars and go completely extinct upon rotation of stage.
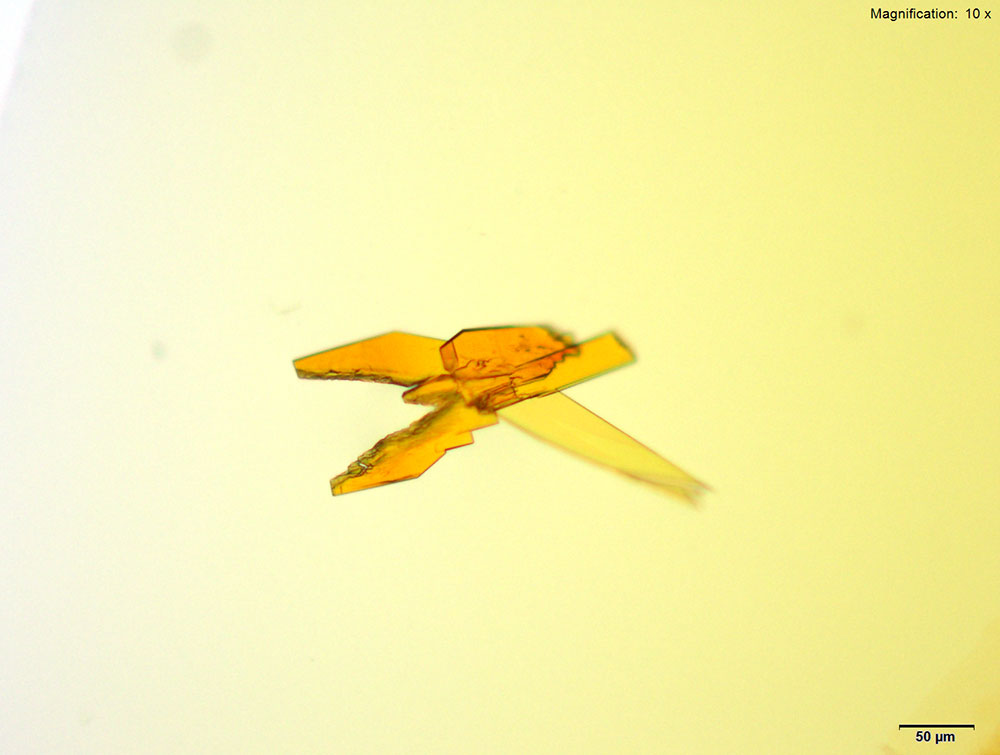
Platinic Chloride (H2PtCl6)
4-Methylbuphedrone dissolved in water or 10% acidic solutions, yellow feathery crystals with serrated edges and composites of tablets are observed. The most characteristic crystals are observed in water. The crystals are very bright under crossed polars.
IR Spectrum - Platinic Chloride (H2PtCl6)
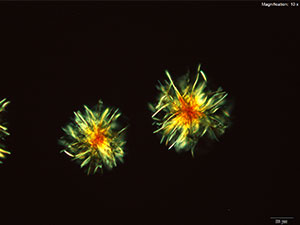
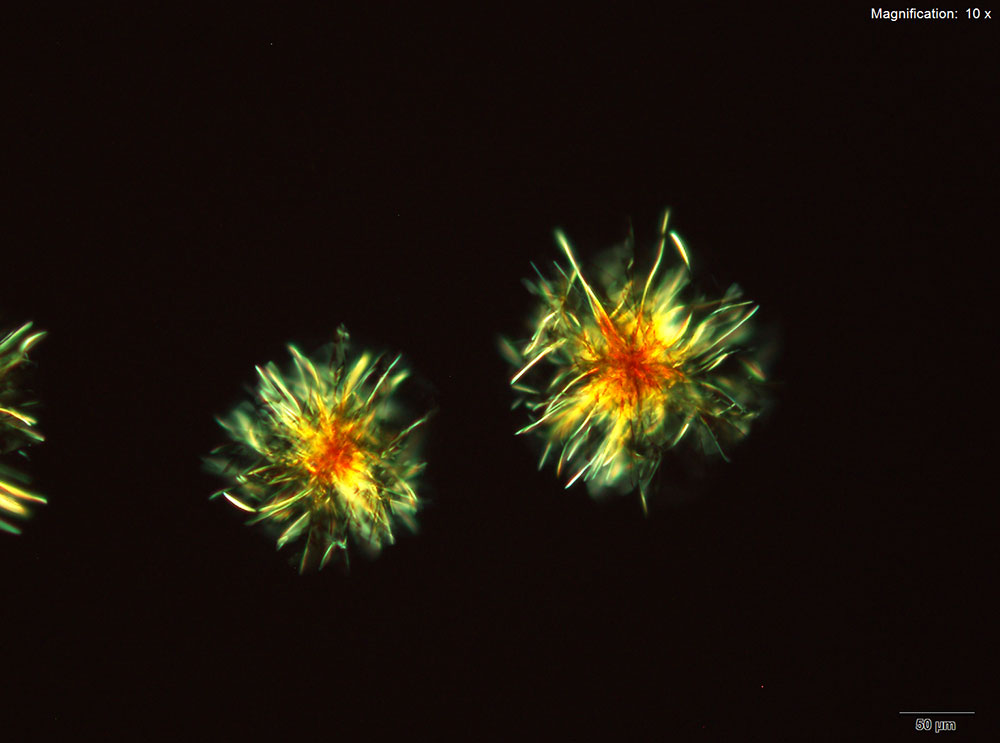
Platinic Bromide (H2PtBr6)
Platinic bromide reagent results in two distinct forms of crystals. The round, tight clusters of light, orange hairs are prominent. The centers of the clusters are very dark and the loose ends are bright under crossed polars. The composites of irregular thick yellow-orange tablets grow very large. These composites are very bright under crossed polars and different parts of the crystal go dark at different times.