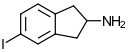
5-IAI
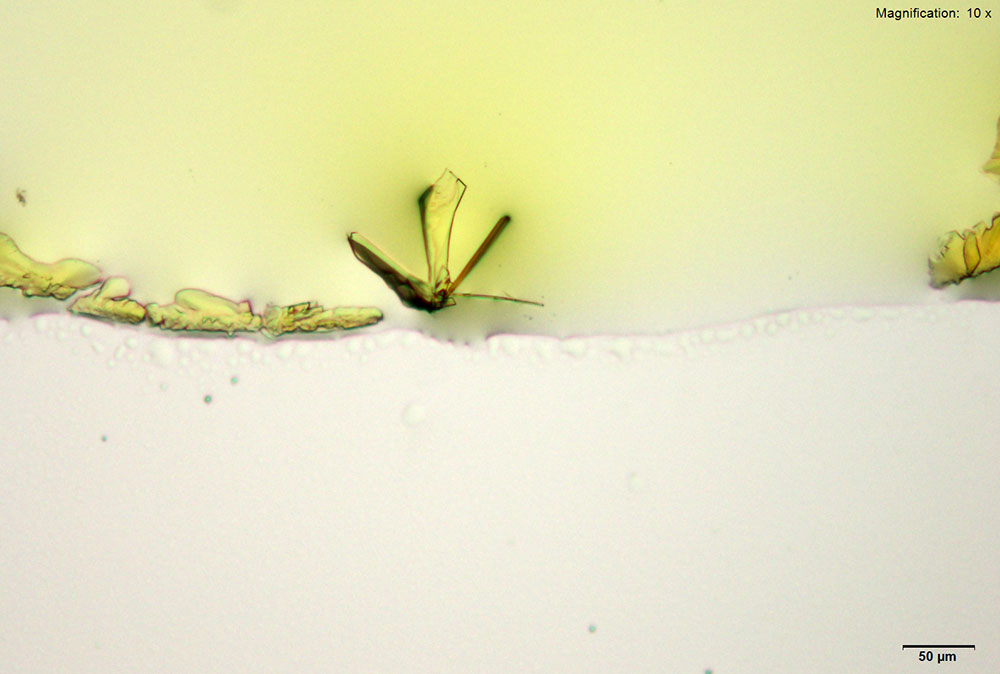
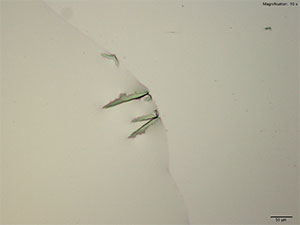
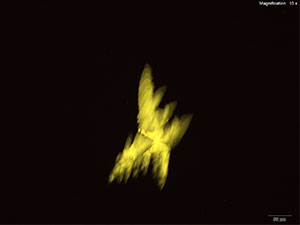
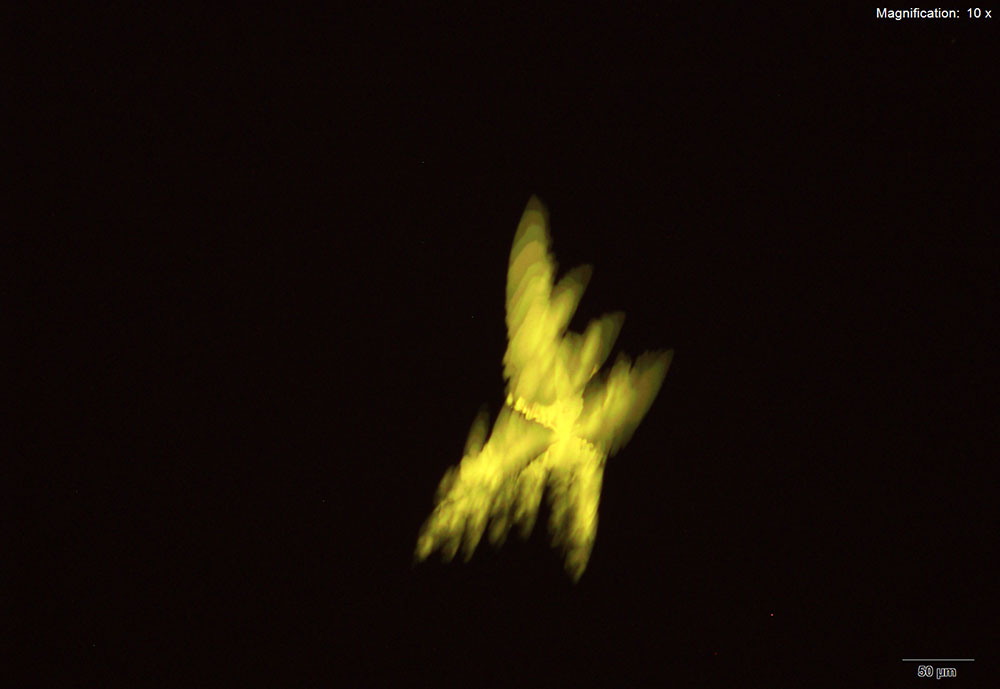
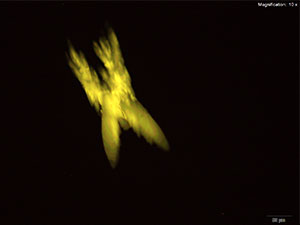
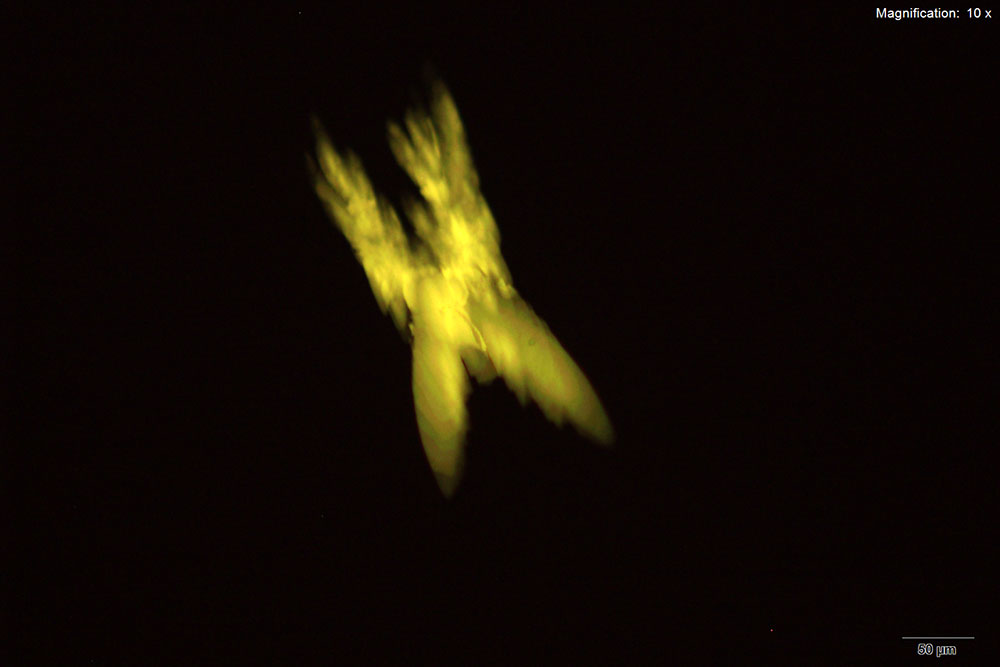
5% Aqueous HAuCl4
5-IAI and gold based reagents all give similar crystals that differ in thickness and color. With aqueous gold chloride reagent form long, thick yellow blades with angled edges. 5μg of the substance dissolved in water when mixed with gold chloride gives the most reliable formation. The blades are very bright under crossed polars and can appear yellow to blue depending on the orientation. Also formed along the edges of the drop is a thick leafy crust that appears a light purple under crossed polars.
5% HAuCl4 in 1:2 concentrated H2SO4: H2O
In the acidic gold chloride reagent, 5-IAI forms distinct long blades with serrated edges. The blades are generally branched with one long blade and shorter branches. The blades appear blue to light purple to white under cross polars.
IR Spectrum - 5% HAuCl4 in 1:2 concentrated H2SO4: H2O
Gold Bromide (HAuBr4)
Gold bromide reagent is the best reagent for this substance. The crystals formed are thick, red blades with serrated edges and sharp triangular heads. The crystals grow reliably and fully in 15 -20 minutes but do not grow in large numbers.
Platinic Chloride (H2PtCl6)
The crystals with platinum based reagents for 5-IAI are not as characteristic with the gold based reagents. The reactions observed with aqueous platinic chloride very small clusters or light leafy blades. Platinic chloride is not a good test for 5-IAI.
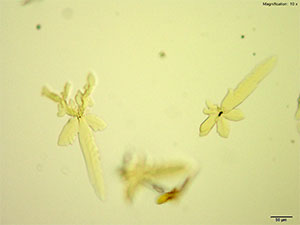
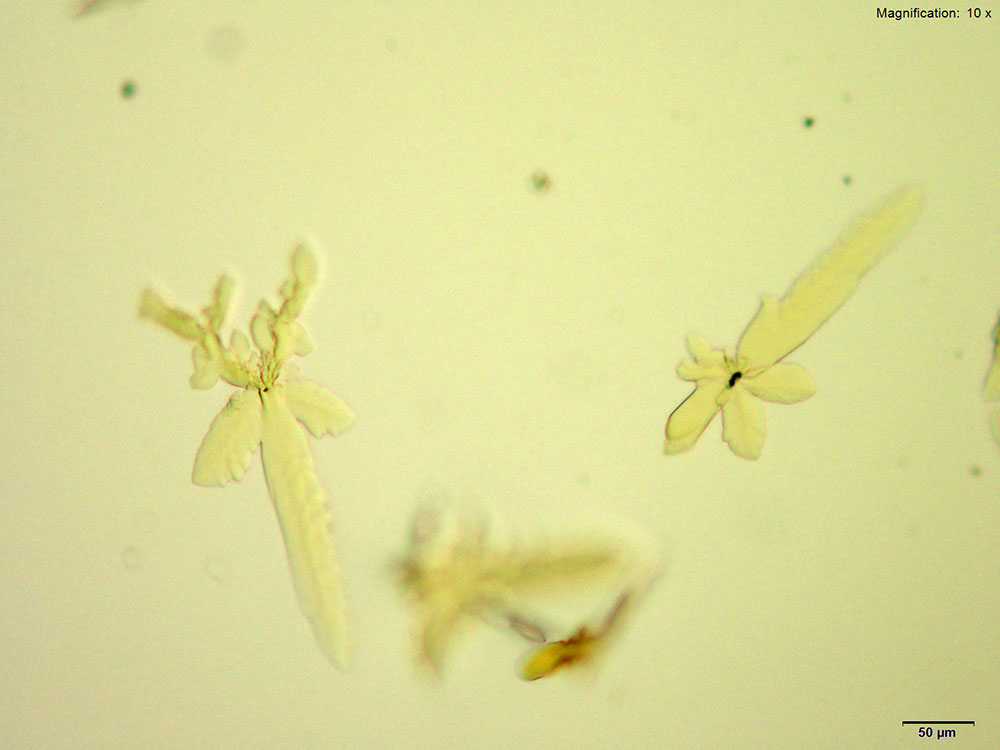
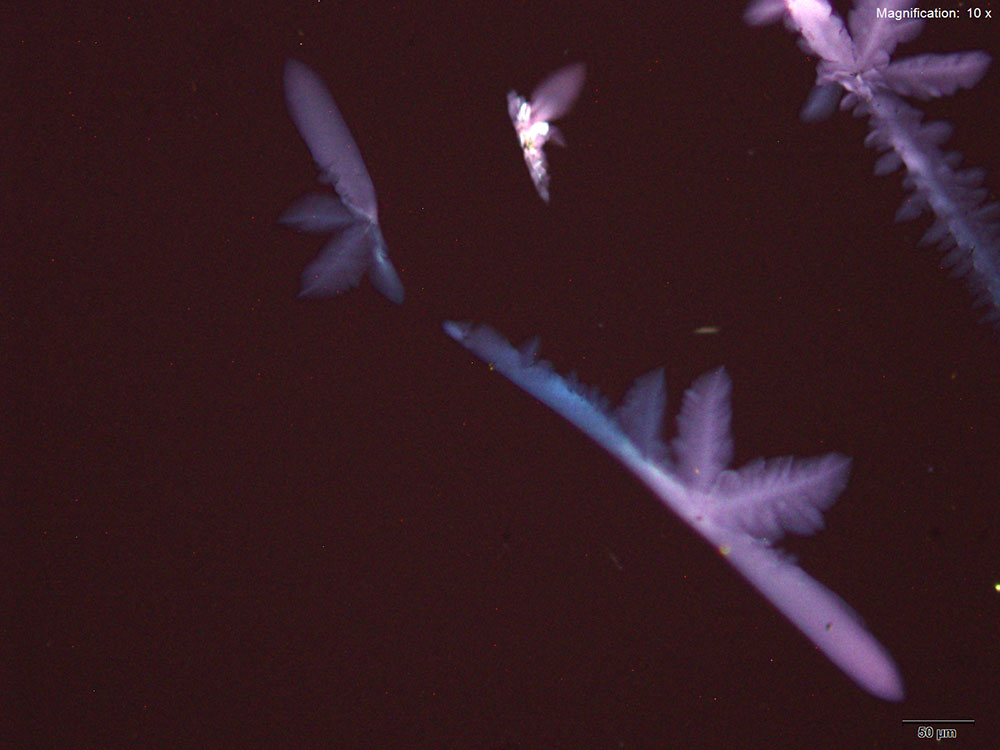
Platinic Bromide (H2PtBr6)
5-IAI and platinic bromide make very light feathery X-shaped faint yellow crystals. The crystals appear white to bright yellow under crossed polars. The crystals are suspended in solution and are best photographed with a cover slip.