Institutional Review Board (IRB)
What is the IRB?
Institutional Review Boards (IRBs) review research studies that involve human participants to ensure that studies: a) follow applicable regulations, b) enact commonly accepted ethical standards, c) meet institutional policies, d) treat participants ethically, and e) appropriately protect research participants from research-related risk. The IRB members include faculty and community members from varying backgrounds who have experience in research, meeting federal regulations for IRB composition. WCU’s IRB includes members who represent the perspectives of scientists, non-scientists, community members not affiliated with the institution, and a prisoner advocate. WCU’s IRB includes members from various colleges and disciplines to offer diverse experiences and perspectives when reviewing IRB applications. (Content adapted from: https://www.hhs.gov/ohrp)
Do I Need IRB?
How do I get help?
- Attend office hours to meet with IRB Co-Chairs or IRB Members
Fall/spring weekly office hours: Listed on the IRB website.
Summer/winter: Please email co-chairs to meet - Contact co-chairs for 1:1 meetings or to attend/present during a class session. mreed3@wcupa.edu or hleaman@wcupa.edu
- Students-work directly with your faculty sponsor through all stages of IRB application development.
- Questions re: Cayuse or IRB application status can be directed to the IRB office at: IRB@wcupa.edu
IRB Initial Application Process & Review Timeline
IRB Applications will be reviewed according to submission date, and typically take 3-4 weeks for review. Investigators should plan 6 weeks to allow time for revisions, if needed.
Application Steps:
- All investigators complete required CITI training and request Cayuse accounts (must use WCU email address when completing Caysue account form).
- Investigator(s) complete the IRB application, including all required materials (application, recruitment messaging, data collection instruments, informed consent and assent documents, letters of support, etc…).
- Student investigators work with their faculty sponsor to review/revise IRB applications before submission.
- Investigator(s) submit and certify the IRB application.
Note: Submission/certification confirms investigators and faculty sponsors have thoroughly reviewed/revised the IRB application.
Note: All investigators must certify the application for it to be received for review. - Applications are received by the IRB Office and assigned to reviewers.
Note: Exempt applications are assigned to one reviewer. Expedited applications are assigned to two reviewers. - Reviewers receive the IRB application via the Cayuse system.
- Reviewers complete an initial review of the application, returning the application to the Primary Investigator(s) within 3 to 4 weeks, if revisions are necessary.
- Investigators address required changes, and submit/certify the application to be returned to the initial reviewers.
- Once all revisions have been accepted, the IRB application will be approved.
- Investigators will be notified of requests for revisions, and approvals, via automated email from Cayuse to their WCU email.
Notes:
- Certification of applications by investigator(s) and/or faculty sponsors confirms that they have reviewed the application in detail prior to submission to the IRB.
- Investigators may check the status of their IRB application after 4 weeks by emailing IRB@wcupa.edu
- Investigators may request to communicate with their IRB reviewers by emailing IRB@wcupa.edu
- Full board applications will require convening of the IRB and timelines for approval may be different than exempt and expedited applications.
- Application status as listed in Cayuse:
Under pre-review (with the IRB office; not yet distributed to reviewers)
Under post-review (with the IRB office; not yet returned to investigators or approved)
Under review (with reviewers)
Unsubmitted (with investigators)
Common Application Problems & Faculty Sponsor Reminders
Common Application Problems:
- Application has not been proofread for consistency.
Investigators should ensure risks/benefits/time commitment/data collection, etc… is consistent throughout application and informed consent. - Informed Consent does not follow federal requirements.
Investigators should use the Informed Consent Creator to ensure all required elements are included in the consent form. Note: The Consent document generated by the Consent Creator must be proofread and edited. - Informed Consent or Recruitment Materials are too lengthy, unclear, the reading level
is above 8th grade.
Investigators are encouraged to check reading levels via online tools and to ensure content is appropriate and accessible for laypersons. - The IRB Application does not follow instructions listed in each section.
Investigators should double check the specific instructions listed above each box in Cayuse.
Faculty Sponsor Reminders:
- Faculty sponsors assume responsibility for their students’ research.
- When certifying an application, modification, or incident report, faculty sponsors are insuring they have reviewed the application in its entirety and are in support of the content submitted by the student.
- Faculty sponsors are responsible for accessing required materials (as per IRB application) in case of federal audit.
Example Materials
Technology Recommendations
The IRB recommends the use of WCU password-protected platforms/programs for data collection, storage, and analysis. PIs are responsible for describing data security, confidentiality, and how data are used by platforms when submitting their IRB application.
Typically Approved Platforms
- WCU’s Qualtrics survey software
- WCU’s Zoom Interviewing platform
- WCU’s Microsoft Suite products
- WCU’s Panopto
- WCU’s Dedoose, SPSS and other WCU-supported data analysis programs
Instructional Videos (Submitting, Modifying, Closing IRB Applications)
Institutional Review Board Members
IRB Members Fall 2025
Co-Chair:
REED, MELISSA
Phone: 610-436-2141
Department: Kinesiology
Address: Sturzebecker HSC 320
E-Mail: mreed3@wcupa.edu
Co-Chair:
LEAMAN, HEATHER
Phone: 610-436-3323
Department: Early & Middle Grades Education
Address: Recitation Hall 203D
E-Mail: hleaman@wcupa.edu
BRUMLEY, LAUREN
Phone: 610-436-2723
Department: Psychology
Address: Main Hall 530
E-mail: lbrumley@wcupa.edu
DI GIOVINE, MICHAEL
Phone: 610-436-2247
Department: Anthropology & Sociology
Address: Old Library 204
E-Mail: mdigiovine@wcupa.edu
GOLMOHAMADI, AMIR
Phone:610-436-2175
Department: Nutrition
Address: SECC 255
E-Mail: agolmohamadi@wcupa.edu
GRUGAN, SHANNON
Phone: 610-436-2659
Department: Criminal Justice
Address: BPMC 515
E-Mail: sgrugan@wcupa.edu
KAHN, SETH
Phone:610-436-1640
Department: English
Address: Main Hall 520
E-Mail: skahn@wcupa.edu
MCCULLOH NAIR, JULIE
Phone: 610-436-2331
Department: Nursing
Address: 930 E. Lincoln HWY 121
E-Mail: jnair@wcupa.edu
OCASIO, KERRIE
Phone: 610-436-3522
Department: Graduate Social Work
Address: Anderson Hall 446
E-Mail: kocasio@wcupa.edu
PARK, INNHWA
Phone: 610-436-2640
Department: Languages & Cultures
Address: Mitchell Hall 232
E-Mail: ipark@wcupa.edu
RAINEAR, ADAM
Phone:610-436-1533
Department: Communication & Media
Address: Wayne Hall 231
E-Mail: arainear@wcupa.edu
STAULTERS, MIMI
Phone:610-436-2398
Department: Educational Leadership & Higher Education Administration
Address: Anderson Hall 218C
E-Mail: mstaulters@wcupa.edu
ZIMMERMAN, REVA
Phone:610-436-2588
Department: Communication Sciences & Disorders
Address: 201 Carter Dr, 418
Email: rzimmerman@wcupa.edu
Non-WCU Community Members:
BRUN, KYLE
E-Mail: KB985201@wcupa.edu, brunk@garnetvalley.org
SPRING 2026 IRB co-chairs office hours (for faculty, staff and students):
Dr. Melissa Reed
Wednesdays 8:30 to 9:30 a.m.
Zoom Link
Dr. Heather Leaman
Tuesday 4-5 p.m.
Zoom Link
College-Wide Office Hours:
All faculty, staff and students are welcome to attend college-wide office hours. Please see the scheduled times and more information here .
Renewals, Modifications, and Closures
Modifications for Cayuse IRB
ALL revisions to WCU IRB approved protocols (even minor revisions such as adding a
new student assistant’s CITI Human Subjects Research training completion report) must
be submitted as a formal modification.
Closures for Cayuse IRB
Upon completion of your project, WCU requires all investigators to close their research
project via Cayuse.
To submit renewals, modifications, or closures:
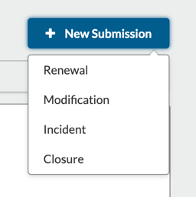
- Investigators open the approved IRB application.
- Investigators select +New Submission to choose renewal, modification, incident, closure.
- Renewals and Modifications are reviewed by the IRB Co-Chairs.
- Investigators should contact the IRB Co-Chairs if they have questions.
Incident Reporting
The Common Rule requires prompt incident reporting. Reports of unanticipated problems involving risks to subjects or others, including breach of data or confidentiality must be reported directly to the IRB as soon as the event is recognized.
Incident reports are filed in Cayuse. Investigators are encouraged to discuss the incident with IRB Co-Chairs prior to or immediately after submitting the incident report. Incident reports must include detailed information about the incident (dates/time), and how the investigators have rectified the incident. Co-Chairs can assist investigators in making swift decisions to rectify incidents and protect human subjects.