Dynamics of Adsorption and Desorption
I have long studied the simplest of surface chemical reactions, the adsorption and thermal desorption of small molecules from surfaces, for example, nitric oxide on platinum, hydrogen on silicon, oxygen on palladium, and methane on platinum. Also I have reviewed stimulated desorption of hydrogen from silicon. A superb review of the dynamics of hydrogen adsorption onto and desorption from silicon has been published by Dürr and Höfer.
These studies attempt to under how chemical bonds are made and broken in the most elemental of surface reactions: that of a molecule approaching a surface and sticking to it or of a molecule detaching from a surface. We also investigate the dynamics of how electronic excitations induced by electron and photon irradiation affect surface chemical reactions.
Adsorption is either activated or nonactivated. In nonactivated adsorption, low energy molecules stick better than high energy molecules. For activated adsorption, a molecule must have a minimum amount of energy before it is able to stick. But it is details of the potential energy surface (PES) that determine what types of energy (kinetic, vibrational, rotational and electronic) are most effective at enhancing activation adsorption or reducing nonactivated adsorption. How the molecule moves over this potential energy surface is what we meant by dynamics. What is the path taken by the molecule and how do the initial conditions of the molecule and the surface affect the sticking probability of the molecule? This is the type of fundamental question asked.
Desorption occurs over the same PES as adsorption with the motions, in a sense, reversed. Thus, adsorption and desorption are related by microscopic reversibility so long as the two process are carried out at the same conditions. In particular, it is important that the coverage of surface bound species (adsorbates) is the same when comparing adsorption and desorption using microscopic reversibility. For hydrogen on silicon this is especially important.
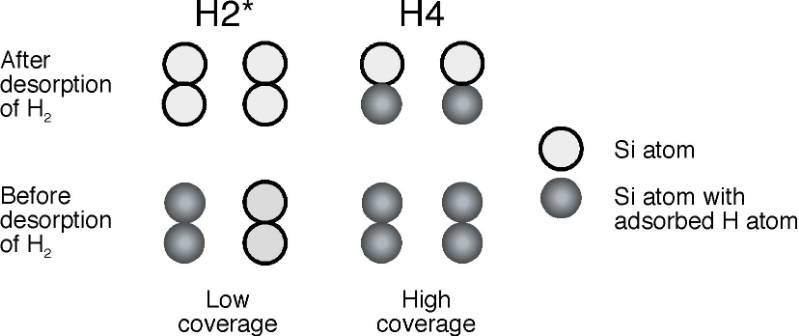
Hydrogen adsorption leads to changes in the silicon surface. The surface structure relaxes and both its geometry and vibrational properties change in response to adsorption. This effect is generally greater on the surfaces of covalent solids such as semiconductors. While such effects may also be important on metal surfaces, the most studied case of hydrogen on copper does not exhibit a similarly strong dependence on coverage. For hydrogen on silicon, the changes introduced are so strong that there appears to be one mechanism that describes dissociative adsorption of H2 into two H atoms on the surface at low coverage (with little adsorbed H on the surface, denoted H2* in the figure below or intradimer desorption) as compared to high coverage (when there is nearly one adsorbed H atom on every surface Si atom, denoted H4 in the figure below or interdimer desorption). The adsorption and desorption of hydrogen is of great interest from a fundamental viewpoint since it is (supposedly) the simplest reaction of a molecule with the most important semiconductor. However, it is also of practical interest because the adsorption and desorption of hydrogen play an important role in the chemical vapor deposition (CVD) of silicon from silanes (such as SiH4and Si2H6), which is important in the integrated circuit industry.
Methane (CH4) is a molecule that is very important in\ a range of catalytic chemistry. Methane is the primary component ofnatural gas and steam reforming of methane over a nickel catalyst is the primary industrial source of hydrogen. We examined the dynamics of methane and ethaneadsorption on metal surfaces using effusive molecular beam techniques. Effusive beams from a Knudsen source have a distribution of rotational, vibrational and translational energy that is described by an equilibrium thermal distribution (a Maxwell-Boltzmann distribution). Methane activated adsorption is quite interesting because it has a very low sticking coefficient and exhibits vibrational mode-specific chemistry. In other words, excitation of the molecule is required to get it to stick and the amount of sticking coefficient enhancement depends on the vibrational mode that is excited. Not all modes are equally active at enhancing sticking. While this is very interesting from a fundamental scientific standpoint, it is still unclear whether the mode specificity is relevant for the kinetics of the industrial process.
